- Gold Periodic Table Mass Number
- Gold Mass Number And Atomic Number
- Gold Atomic Mass And Number
- Gold Mass Number Rounded
- Gold Atomic Mass Number
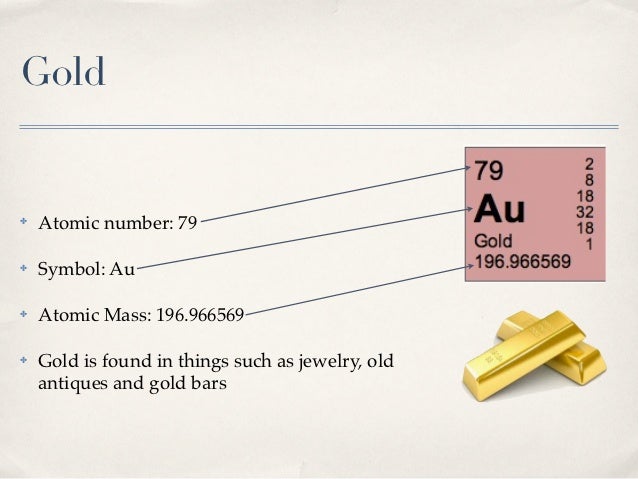
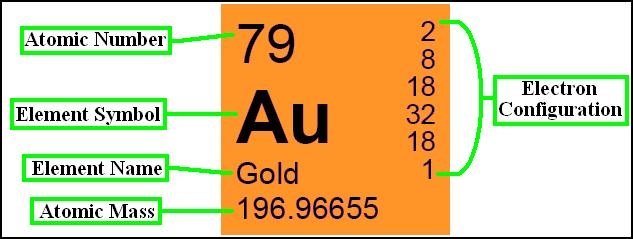
Au, which is also its only naturally occurring isotope, so gold is both a mononuclidic and monoisotopic element. Thirty-six radioisotopes have been synthesized, ranging in atomic mass from 169 to 205. The most stable of these is 195 Au with a half-life of 186.1 days. Gold, isotope of mass 195 Please visit the Gold element page for information specific to the chemical element of the periodic table. Name: Gold Symbol: Au Atomic Number: 79 Atomic Mass: 196.96655 amu Melting Point: 1064.43 °C (1337.5801 K, 1947.9741 °F) Boiling Point: 2807.0 °C (3080.15 K, 5084.6 °F) Number of Protons/Electrons: 79 Number of Neutrons: 118 Classification: Transition Metal Crystal Structure: Cubic Density @ 293 K: 19.32 g/cm 3 Color: Gold Atomic Structure. Chemical symbol for Gold is Au. Number of protons in Gold is 79. Atomic weight of Gold is 196.966569 u or g/mol. Melting point of Gold is 1064,4 °C and its the boiling point is 2940 °C. » Boiling Point » Melting Point » Abundant » State at STP » Discovery Year.
What are the numbers of the subatomic particles in gold?
1 Answer
A Gold (Au) atom has 79 protons and 79 electrons. A typical gold atom has 118 neutrons, though there are 18 other radioisotopes discovered so far.
From the periodic table, a gold atom is represented by
79 is its charge (atomic number), which is both its proton number and electron number.
197 is its mass (atomic mass), which is the sum of its proton number and neutron number. The neutron number can be calculated by subtracting the proton mass from the total mass.

The mass of the electrons does not contribute significantly to the total mass of the atom and therefore can be ignored.
Related questions
Our editors will review what you’ve submitted and determine whether to revise the article.
Gold Periodic Table Mass Number
Join Britannica's Publishing Partner ProgramGold Mass Number And Atomic Number
and our community of experts to gain a global audience for your work!Gold Atomic Mass And Number
Gold (Au), chemical element, a dense lustrous yellow preciousmetal of Group 11 (Ib), Period 6, of the periodic table. Gold has several qualities that have made it exceptionally valuable throughout history. It is attractive in colour and brightness, durable to the point of virtual indestructibility, highly malleable, and usually found in nature in a comparatively pure form. The history of gold is unequaled by that of any other metal because of its perceived value from earliest times.
| atomic number | 79 |
|---|---|
| atomic weight | 196.96657 |
| melting point | 1,063 °C (1,945 °F) |
| boiling point | 2,966 °C (5,371 °F) |
| specific gravity | 19.3 at 20 °C (68 °F) |
| oxidation states | +1, +3 |
| electron configuration | [Xe]4f145d106s1 |
Gold Mass Number Rounded
Gold Atomic Mass Number
- key people
- related topics
